
Clinical Pharmacology that Accelerates Global Drug Development
Crystal Bio Solutions delivers integrated biometrics, pharmacometrics, and modeling and simulation to focus your development, reduce risk, and speed decisions. Our global team supports early nonclinical translation through late-stage clinical programs across various drug modalities. Services include SAS programming, CDISC deliverables, NCA, population PK/PD, QSP, and inspection-ready documents for regulatory submission. A2PG is fully integrated into our global clinical pharmacology group.
End-to-end SAS programming with CDISC compliance: SEND for nonclinical, SDTM and ADaM for clinical, TLF packages, define.xml, reviewer guides, and validation with Pinnacle 21 to support IND, CTA, and BLA/MAA submissions.
Hands-on guidance from senior scientists for study design, SAP alignment, decision frameworks, and authority interactions. Outputs are inspection-ready and aligned with ICH expectations.
 Model Informed Drug Development (MIDD)
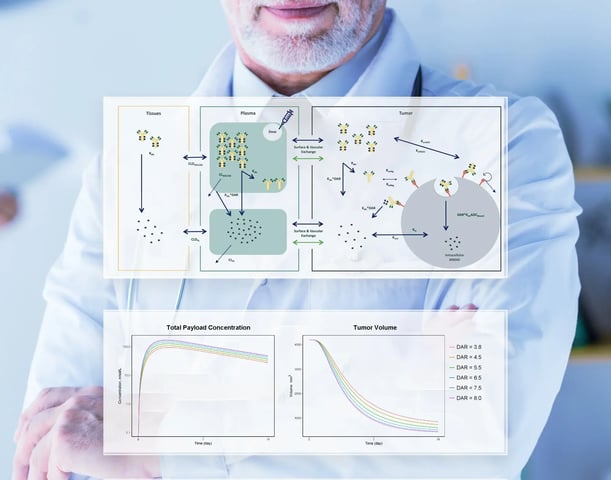
We build quantitative pharmacostatistical models that describe PK, efficacy, and safety, then run simulations to compare clinical scenarios. Outputs inform first-in-human dose, adaptive or enrichment strategies, endpoint selection, and patient or indication prioritization. We also prepare briefing packages and responses for authority meetings and support NDA, BLA, and MAA filings.
Model Informed Drug Development (MIDD)
We build quantitative pharmacostatistical models that describe PK, efficacy, and safety, then run simulations to compare clinical scenarios. Outputs inform first-in-human dose, adaptive or enrichment strategies, endpoint selection, and patient or indication prioritization. We also prepare briefing packages and responses for authority meetings and support NDA, BLA, and MAA filings.
 Global Drug Development and Launch
Teams in the US, China, and EU coordinate with our regulatory, clinical, and bioanalytical groups to deliver coherent global plans. Services cover regional strategy and bridging, labeling and claims planning, and launch-readiness activities, so sponsors move from pivotal data to approval with confidence.
Global Drug Development and Launch
Teams in the US, China, and EU coordinate with our regulatory, clinical, and bioanalytical groups to deliver coherent global plans. Services cover regional strategy and bridging, labeling and claims planning, and launch-readiness activities, so sponsors move from pivotal data to approval with confidence.



