Dec 15, 2025
Sterility Assays in Drug and Device Manufacturing
Sterility and endotoxin assays are essential GMP tests ensuring drug and device safety, regulatory compliance, and reliable release across biomanufacturing workflows.
 \
\
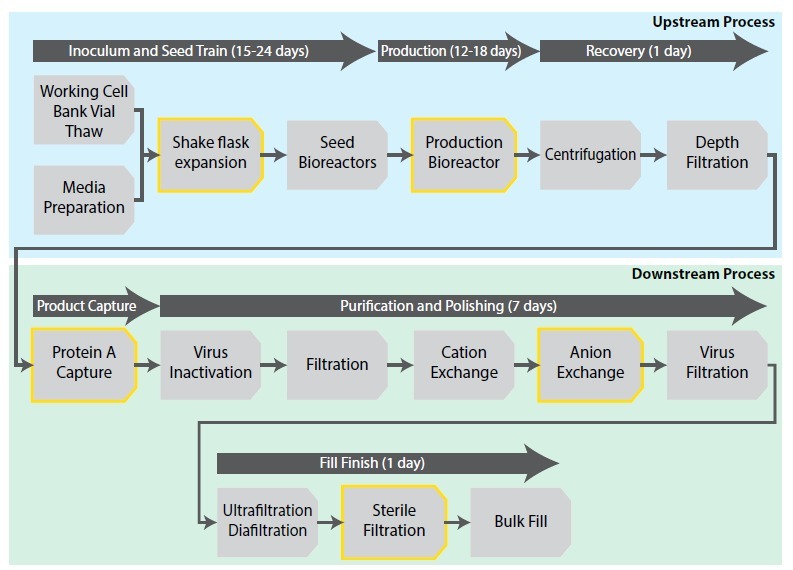
Figure 1. Process flow diagram of a monoclonal Antibody biomanufacturing process. Yellow highlights denote the checkpoints for “pulling” samples for microbial and endotoxin testing. (adapted from https://www.intechopen.com/chapters/84864)
Why Are Sterility Assessments Important?
In the biomanufacturing industry, Critical Quality Attributes (CQA) are essential standards that must be met before biopharmaceuticals or medical devices can be released for patient use. As a fundamental component of CQA for a Drug Product (DP), manufacturers are required to conduct rigorous testing to ensure compliance with regulatory limits for adventitious agents. These agents include microorganisms such as bacteria and fungi, potential viruses, endotoxins, and mycoplasma.
Sterility testing constitutes a mandatory and vital aspect of the pharmaceutical industry. Non-compliance with sterility regulations, particularly concerning the release of DPs, can result in severe, even fatal, consequences. Sterility testing is a cornerstone of Good Manufacturing Practice (GMP) microbiology requirements and is employed to verify that DPs are free from viable microorganisms or associated substances, such as endotoxins, prior to their administration to patients.
This testing is critical to ensuring the safety and efficacy of pharmaceutical products, safeguarding patient health, and maintaining regulatory compliance.
Regulatory Guidelines and Relevance
According to the guidelines specified in the US Pharmacopeia1,2,3,4, biotherapeutics and medical devices must undergo microbial screening for bacteria and fungi prior to release. For products where sterilization is feasible, typically therapeutic molecules via membrane filtration for proteins and other biomolecules, and devices through methods such as ethylene oxide treatment, gamma ray irradiation, or steamt-sterility testing is conducted post-sterilization, even if they are not produced under aseptic conditions.
The FDA provides specific guidelines for microbial and endotoxin limits for monoclonal antibodies produced using bacterial or yeast systems during manufacturing. In many instances, samples or Drug Substances (DS) are collected pre-sterilization and analyzed for bioburden, which measures the total number of viable microorganisms on surfaces like medical devices, containers, or components.
This assessment is crucial for evaluating manufacturing procedures prior to sterilization and serves as an indicator of compliance with Good Manufacturing Practices (GMP). Although pre-sterilization bioburden studies are not used as a criterion for release and do not directly impact subsequent manufacturing steps, they are essential for ensuring consistency and a reasonable level of sterility within the manufacturing processes.
Consequently, many manufacturing organizations perform in-process DS microbial and endotoxin testing as part of internal Quality Control to monitor and verify the sterility and cleanliness of the manufacturing process, as depicted in the figure 1. This proactive approach is critical in maintaining high standards of product safety and quality throughout the production lifecycle.
Traditional compendial tests
Sterility testing for drug substances (DS) has traditionally employed methods such as the Tryptic Soy Agar plate method (for aqueous samples ranging from 0.1 mL to 2 mL) or the Membrane Filtration method (for aqueous samples greater than 2 mL), and involves the use of both aerobic (e.g., soybean casein digest medium) and anaerobic (e.g., fluid thioglycolate medium) microbial growth media.
These tests generally span at least 14 days, facilitating the detection of fastidious microbes. Positive controls, typically inoculated at levels of <=100 CFU, usually yield detectable growth for bacteria and fungi within 5 and 7 days, respectively.
However, an additional week is recommended by the FDA for the release6 of drug products (DP) manufactured through aseptic processing, particularly for cell and gene therapy products that involve cells which cannot undergo sterilization.
During such tests, if the positive control fails to exhibit microbial growth, or if contamination is detected in the DS/DP samples and exceeds permissible limits as outlined in the Table, the media must be re-examined and the test potentially repeated. This could involve repeating the entire 14-day process to determine whether the failure was due to media conditions unsupportive of microbial growth or external contamination during sterility testing. Such repetitions often represent a significant bottleneck in the release process for most products.

List of specified microorganisms for which acceptance criteria are set. (From USP < 1111>)
Alternative to Compendial Methods
To relieve the timeline-associated stress linked with the release of potentially life-saving treatments, such as biotherapeutic molecules and cell and gene therapy products, the FDA has outlined guidelines for validating new sterility tests. These guidelines adhere to protocols established in USP Chapter 1223, "Validation of Alternative Microbiological Methods." The guidelines permit the use of rapid sterility tests, including the 7-day assays using automated microbial detection systems like BacT/Alert (Biomerieux) or BACTEC (BD Biosciences).
These systems typically involve traditional methods of inoculating media. Additionally, molecular techniques such as quantitative PCR, exemplified by the SteriSEQ system (Thermo Fisher), are also endorsed. These alternative methods are designed to expedite the testing process while ensuring compliance with regulatory standards, thereby facilitating faster market access for critical healthcare products.
Safety assessment of a DP via Endotoxin measurement
In addition to standard sterility assays, the FDA requires an independent evaluation for endotoxins, as a drug product (DP) that meets sterility standards per USP <1111> may not necessarily be pyrogen-free. Endotoxins, which are lipopolysaccharides found in the cell walls of Gram-negative bacteria, are significant pyrogens. Despite sterilization processes that eliminate bacteria in biotherapeutic molecules, or the absence of bacteria in initial leukopak materials from patients or donors in cell and gene therapy, endotoxins may still be present.
While microbial testing falls under sterility checks, endotoxin testing is crucial for assessing the "safety" of a DP. Exposure to endotoxins can lead to severe adverse effects such as fever, organ failure, septic shock, or even death if introduced into the bloodstream or spinal fluid beyond permissible levels. USP <85> outlines the methodology for determining allowable endotoxin limits for any DP and describes various detection methods, including the use of Limulus amoebocyte lysate (LAL) derived from horseshoe crabs (Limulus polyphemus or Tachypleus tridentatus). These methods can be implemented in rapid, cartridge-based systems or through plate-based kinetic turbidimetric or chromogenic techniques.
Further emphasizing safety, the FDA Guidance for Industry: Pyrogen and Endotoxins 2012 mandates that all injectable and implantable drug products and devices undergo testing to ensure endotoxin levels are within the specified safe range before being released for patient use. This directive is integral to ensuring that products are not only sterile but also free from pyrogenic contaminants that could compromise patient health.
Conclusion
Sterility assays and endotoxin assessments are critical components of the safety evaluation process for drug manufacturing, particularly in the fields of biotherapeutics for molecules and devices, and cell and gene therapy. It is essential that both media and drug products (DP) undergo independent evaluations for matrix interference, signal suppression, and recovery during the drug development stage. This ensures that a robust, validated method is in place, which can then be seamlessly transferred to the Quality Control (QC) laboratory during the manufacturing process. Such preemptive measures are vital for maintaining the integrity of the manufacturing workflow and ensuring that the final products meet all regulatory safety standards before reaching the market.
This approach not only safeguards patient health but also streamlines regulatory compliance and enhances the overall efficiency of drug production.
Capabilities at Crystal Bio Solutions
Crystal Bio Solutions, a Contract Research Organization (CRO), specializes in providing comprehensive method development services for sterility and endotoxin assessment assays. The organization validates rapid 5–7-day sterility assessments against the traditional 14-day compendial testing for drug products (DP), alongside designing and executing suitable methods for endotoxin assessment. Following method development, Crystal Bio ensures a seamless technology transfer to GMP suites. This approach eliminates the need for an Analytical Method Transfer department at the prospective client or Intellectual Property (IP) holder's site.
This strategy not only reduces the costs associated with training personnel but also significantly saves time - a critical factor, particularly during the release phase of a DP. Such efficiencies are crucial for accelerating the time-to-market for new therapies, enhancing the overall productivity of the drug development and release process.


